Addressing a poorly served stage of the cancer patient journey

Many technologies support cancer screening, diagnosis, and early-stage treatment, but only AIQ enables optimization of late-stage care
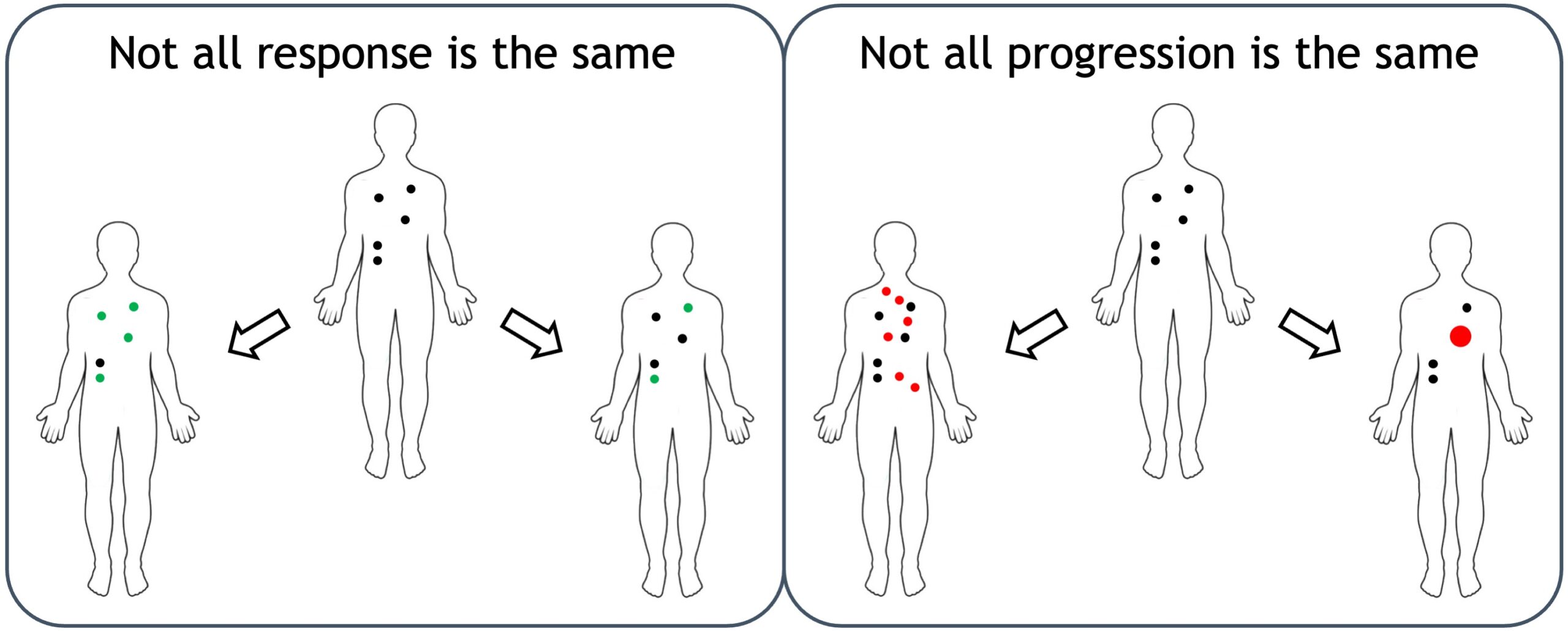
For late-stage cancer, each individual lesion responds differently to therapy
TRAQinform IQ Technology
TRAQinform IQ is a software medical device that provides novel information about quantification of change in regions of interest over time. Oncologists/clinicians use this technology to more precisely adjust therapies and optimize clinical outcomes.
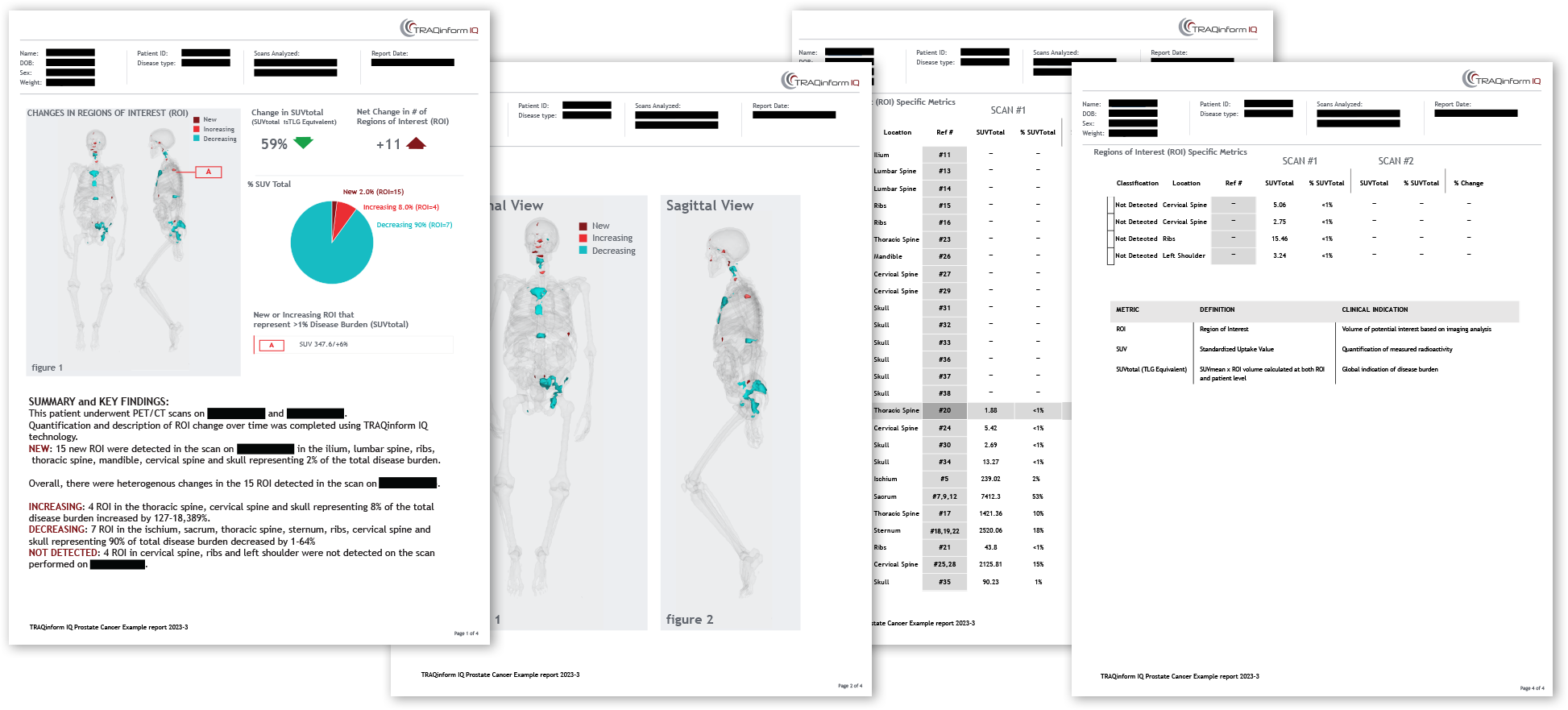
TRAQinform IQ Report
Comparison to Standard of Care

Easy integration into existing workflows
 TRAQinform IQ uses serial radiographic images (e.g., PET, CT) |
 |
 Images are uploaded via the cloud to AIQ solutions (HIPAA compliant) |
 |
 Software that provides novel, quantitative and spatial information about each region of interest |
 |
 A comprehensive report is generated and delivered to the oncologist |
 |
 Oncologists can use the information to tailor treatment regimens and optimize outcomes |
Interested in using TRAQinform IQ at your hospital?
1: Seyfried TN, Huysentruyt LC. On the Origin of Cancer Metastasis. Crit Rev Oncog. 2013;18(1-2).
2: Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1302-1312.
3: Lokre, O., Perk, T.G., Weisman, A.J. et al. Quantitative evaluation of lesion response heterogeneity for superior prognostication of clinical outcome. Eur J Nucl Med Mol Imaging. 2024.
4: Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328-37.
5: Harmon SA, Perk T, Lin C, et al. Quantitative Assessment of Early [18F]Sodium Fluoride Positron Emission Tomography/Computed Tomography Response to Treatment in Men With Metastatic Prostate Cancer to Bone. J Clin Oncol. 2017;35(24):2829-2837, including unpublished supplemental data analysis.
6: Kyriakopoulos CE, Heath EI, Ferrari A, et al. Exploring Spatial-Temporal Changes in 18F-Sodium Fluoride PET/CT and Circulating Tumor Cells in Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide. J Clin Oncol. 2020;38(31):3662-3671.