Myeloma News
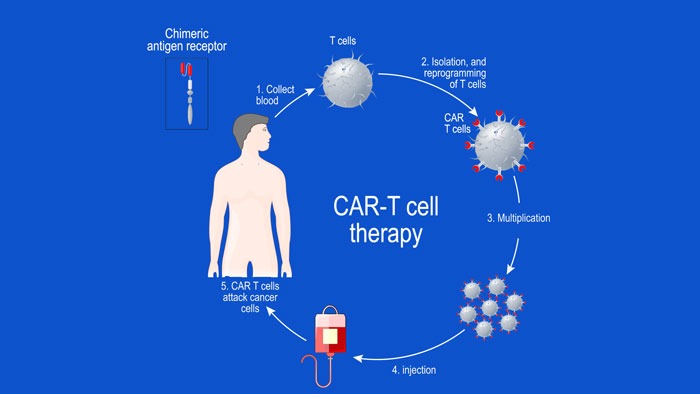
Targeting myeloma essential genes using NOT Gated CAR T-cells, a computational approach
Elotuzumab Efficacy May Suffer if Given After Daratumumab in Multiple Myeloma
Source: Myeloma – Hematology Advisor
Overall survival (OS) and other efficacy outcomes were worse among patients with multiple myeloma (MM) treated with elotuzumab-based regimens after having received daratumumab compared with patients who did not have prior daratumumab, according to a retrospective study published in the Annals of Hematology. However, this decrease in efficacy was somewhat attenuated when elotuzumab was administered 180 days or more after daratumumab or if given nonsequentially.
The multicenter study analyzed data from 127 patients with MM who received elotuzumab from the Kansai Myeloma Forum registry. The primary endpoint was OS and secondary endpoints included time to next treatment (TTNT) and best response.
The median age at diagnosis was 67 and the median age at elotuzumab use was 71. The median number of prior therapies in the daratumumab cohort was 6 (range, 2-13) and was 5 (range, 1-13) in the no daratumumab cohort. Nearly all patients had prior lenalidomide, and 68% and 74% in the cohorts with or without daratumumab, respectively, had prior pomalidomide exposure.
OS was significantly longer in the no-daratumumab cohort, with a 1-year rate of 82.1% compared with 68.7% in the daratumumab cohort (P =.027).TTNT was also longer in the group without prior daratumumab at 188 days compared with 112 days in the daratumumab group (P =.024).
Best response rates were higher in the no-daratumumab group compared with the daratumumab group (P <.01), including the rates of complete response (11.3% vs 2.1%), very good partial response (11.3% vs 4.3%), and partial remission (27.5% vs 12.8%).
Among patients who were treated with daratumumab before receiving elotuzumab, nonsequential use was associated with better outcomes. The 1-year OS was 78.8% in the nonsequential group compared with 50.0% in the sequential group (P =.023). The median TTNT was longer in the nonsequential cohort at 217 days compared with 42 days in the sequential cohort (P =.031).
Best response rates were also higher with nonsequential vs sequential use for very good partial response (3.0% vs 0%) and partial remission (30.3% vs 14.3%), but this was not statistically significant (P =.120).
A longer interval of 180 or more days between the use of daratumumab and elotuzumab was associated with better outcomes than a shorter duration. The 1-year OS was 80.0% in the long interval group compared with 62.5% in the short interval group, but this did not reach statistical significance (P =.084). The mediant TTNT was 204 days and 90 days in the long and short interval groups, respectively (P =.012).
“We demonstrated that OS, TTNT, and best response were worse in the cohort treated with elotuzumab if daratumumab had been given beforehand,” the authors wrote in their report.
Disclosures: Some of the study authors declared affiliations with biotech, pharmaceutical, or device companies. Please see the original reference for a full list of disclosures.
Nurse Insights for Managing CRS in Patients With RRMM Receiving Teclistamab
Source: Myeloma – Hematology Advisor
As frontline caregivers, nurses are instrumental in promptly identifying and managing CRS in patients undergoing therapy with teclistamab. By leveraging insights from both the MajesTEC-1 trial and real-world nursing practice, nurses can enhance their ability to diagnose, monitor, and manage CRS effectively.
Cytokine release syndrome (CRS), a systemic inflammatory response commonly observed with T-cell redirecting therapies, presents challenges in patient management. Drawing from the experience of the MajesTEC-1 clinical trial (ClinicalTrials.gov Identifier: NCT04557098) and real-life nursing practice, essential guidance for nurses involved in the care of patients receiving teclistamab for relapse/refractory multiple myeloma (RRMM) was published in Seminars in Oncology Nursing.
Teclistamab, a bispecific antibody, targets CD3 on the surface of T cells and B-cell maturation antigen (BCMA) on the surface of MM cells. It is the first BCMA×CD3 bispecific antibody approved for the treatment of RRMM exposed to an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody (triple-class exposed).
Despite a high incidence of CRS among patients in the trial, the majority of cases were low grade, resolved without treatment discontinuation, and aligned with supportive measures employed in real-life settings.
Insights were drawn from the experience of the MajesTEC-1 clinical trial, a phase 1/2 study of teclistamab in heavily pretreated patients with RRMM and from real-life nursing practice.
In MajesTEC-1, patients were carefully monitored for early signs and symptoms of CRS to mitigate the risk of high-grade CRS. Additionally, a survey of nurses from several study sites provided further insights into nursing best practices for CRS management from academic institutions across 3 countries.
In total, 72% of patients treated with teclistamab experienced CRS, predominantly low grade. CRS was managed with tocilizumab, intravenous fluids, low-flow oxygen therapy, and/or steroids. Notably, all cases resolved without treatment discontinuation.
“Key takeaways include the importance of recognizing and excluding infection as a differential diagnosis to ensure CRS is properly diagnosed and treated, as well as the benefits of partnering with patients and physicians to recognize and report CRS symptoms early, which is crucial for timely intervention,” wrote the authors.
In summary, this review underscores the pivotal role of nurses in recognizing and managing CRS in patients receiving teclistamab. By integrating insights from clinical trials and real-world practice, nurses can optimize patient care and outcomes in the challenging landscape of CRS management.
Disclaimer: This research was supported by Janssen Scientific Affairs, LLC. Some study authors declared affiliations with biotech, pharmaceutical, and/or device companies. Please see the original article for the full list of disclosures.
Clinical Benefits, But Not Improved ORR, Seen With KPd in Lenalidomide-Refractory Multiple Myeloma
Source: Myeloma – Hematology Advisor
Carfilzomib plus pomalidomide and dexamethasone (KPd) failed to meet the primary endpoint of overall response rate (ORR) among patients with early relapsed/refractory multiple myeloma (RRMM) who were lenalidomide-refractory, according to the results of the phase 2 SELECT trial published in the journal Leukemia & Lymphoma. However, the researchers observed other clinical benefits with KPd, including progression-free survival (PFS) and overall survival (OS).
In the open-label SELECT study (ClinicalTrials.gov Identifier: NCT04191616), researchers treated 52 patients with RRMM who had received 1-2 prior lines of therapy with KPd. The primary endpoint was ORR and secondary endpoints included minimal residual disease (MRD) negativity (10-5) at 12 months, duration of response (DOR), time to response (TTR), PFS, and OS.
At baseline, the median age was 68 years, 54% of patients were female, 90% were White, and 8% were Hispanic/Latino. There were 38% of patients with high-risk cytogenetics, and 58% had a prior transplant. All patients were refractory to lenalidomide and 37% were triple-refractory.
There were 58% of patients who demonstrated an overall response, including 6% with a complete response and 35% with a very good partial response. There were 3.8% of patients who achieved MRD negativity at 12 months and 9.6% who achieved it at any time. The median duration of response was 20.3 months and the median TTR was 1.0 months.
During a median follow-up of 15.5 months, the median PFS was 11.1 months and the 1- and 2-year PFS rates were 47% and 33%, respectively. The median OS was 18.8 months during a median follow-up of 16.6 months. The 1- and 2-year OS was 59% and 47%, respectively.
Grade 3 or higher treatment-emergent adverse events (TEAEs) occurred in 67% of patients, with the most common being hematologic. The most common nonhematologic grade 3 or higher TEAEs included acute kidney injury, rash, asthenia, and respiratory failure. There were no treatment-related deaths.
“KPd may be considered an attractive treatment option for an increasing number of patients with relapsed and multidrug-resistant MM,” the study authors wrote in their report. “With lenalidomide as [a standard of care] and increasing use of anti-CD38 mAbs in the frontline setting, effective treatment combinations for lenalidomide-refractory and CD38-refractory patients may be of increasing importance.”
Disclosures: This study was supported by Amgen. Please see the original reference for a full list of disclosures.
Daratumumab-based quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma with high cytogenetic risk
Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial
Single-cell multiomic dissection of response and resistance to chimeric antigen receptor T cells against BCMA in relapsed multiple myeloma
Practical Considerations of T-Cell Engagers in the Management of Relapsed/Refractory Multiple Myeloma
Source: Pharmacy Times articles
Integration of these agents requires supportive care considerations for cytokine release syndrome, neurotoxicity, infection, and antigen-specific toxicities to ensure optimal care of patients with multiple myeloma receiving T-cell–engaging therapies.
Read More
Single Question Can Predict Treatment Discontinuation in MM Patients
Source: Myeloma – Hematology Advisor
A single question can be used to predict early treatment discontinuation in patients with multiple myeloma (MM), according to research published in JAMA Network Open.
Researchers found that a patient’s response to item 5 from the Functional Assessment of Cancer Therapy-General General Physical Wellbeing Scale (GP5) was significantly associated with subsequent treatment discontinuation because of adverse events (AEs).
Item GP5 is “I am bothered by side effects of treatment,” and responses include “very much,” “quite a bit,” “somewhat,” “a little bit,” and “not at all.”
The researchers evaluated GP5 responses from 1058 patients with MM enrolled in the E1A11 trial (ClinicalTrials.gov Identifier: NCT01863550).
The trial enrolled patients with newly diagnosed, standard-risk MM. They were randomly assigned to receive either bortezomib, lenalidomide, and dexamethasone (n=531) or carfilzomib, lenalidomide, and dexamethasone (n=527) as induction, followed by maintenance. Only data from the induction phase were used in this analysis.
The patients answered GP5 at baseline, 1 month, 2.8 months, and 5.5 months. Responses of “very much” or “quite a bit” were categorized as “high bother” from AEs. Responses of “somewhat,” “a little bit,” and “not at all” were categorized as “low bother” from AEs.
In an adjusted analysis, the odds of early treatment discontinuation were higher for patients with high bother from AEs at 1 month than for those with low bother from AEs at 1 month (adjusted odds ratio [aOR], 2.20; 95% CI, 1.25-3.89).
The odds of early treatment discontinuation for patients with high bother from AEs increased at 2.8 months (aOR, 3.41; 95% CI, 2.01-5.80) and at 5.5 months (aOR, 4.66; 95% CI, 1.69-12.83).
The researchers also noted that the baseline-adjusted, maximum GP5 value while undergoing treatment was significantly associated with early treatment discontinuation for patients with high bother from AEs (aOR, 1.54; 95% CI, 1.04-2.30).
Patients had higher odds of early treatment discontinuation if they experienced worsening of 2 or more GP5 categories from baseline to 2.8 months (aOR, 3.02; 95% CI, 1.64-5.54) and to 5.5 months (aOR, 5.49; 95%CI, 1.45-20.76). There were no such associations at 1 month or for worsening of only 1 category.
“These results point to the usefulness of GP5 as an overall AE impact measure for patients receiving treatment,” the researchers wrote. “Because it is very brief, GP5 may be very useful as a low-burden way to track treatment tolerability over time, both in the context of clinical trials and in routine care.”
Disclosures: One study author declared affiliations with biotech, pharmaceutical, and/or device companies. Please see the original reference for a full list of disclosures.
TRIM33 loss in multiple myeloma is associated with genomic instability and sensitivity to PARP inhibitors
Recent Publications
Decreased RNA-binding protein heterogeneous nuclear ribonucleoprotein U improves multiple myeloma sensitivity to lenalidomide
Br J Haematol. 2024 Apr 29. doi: 10.1111/bjh.19468. Online ahead of print. ABSTRACT Multiple myeloma (MM) is an incurable plasma cell cancer in the bone marrow. Immunomodulatory drugs, such as lenalidomide (LEN) and pomalidomide, are backbone agents in MM treatment, and LEN resistance is commonly...
A genetic variant study of bortezomib-induced peripheral neuropathy in Chinese multiple myeloma patients
Oncol Res. 2024 Apr 23;32(5):955-963. doi: 10.32604/or.2023.043922. eCollection 2024. ABSTRACT BACKGROUND: Bortezomib results in peripheral neuropathy (PN) in approximately 50% of patients, during multiple myeloma (MM) treatment, a complication known as Bortezomib-induced peripheral neuropathy...
Assessment of prolonged proteasome inhibition through ixazomib-based oral regimen on newly diagnosed and first-relapsed multiple myeloma: A real-world Chinese cohort study
Cancer Med. 2024 May;13(9):e7177. doi: 10.1002/cam4.7177. ABSTRACT OBJECTIVE: To evaluate the effectiveness, safety, and convenience of in-class transition (iCT) from intravenous bortezomib-based induction to ixazomib-based oral regimens. METHODS: This retrospective real-world study was conducted...
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of elranatamab for multiple myeloma, CPX-351 for myeloid leukemia, inotuzumab ozogamicin for lymphoblastic leukemia, capivasertib for breast cancer, and zolbetuximab for gastric cancer in Japan
Int J Clin Oncol. 2024 Apr 30. doi: 10.1007/s10147-024-02531-1. Online ahead of print. NO ABSTRACT PMID:38687406 | DOI:10.1007/s10147-024-02531-1
T cell characteristics impact response and resistance to T cell-redirecting bispecific antibodies in multiple myeloma
Clin Cancer Res. 2024 Apr 30. doi: 10.1158/1078-0432.CCR-23-3333. Online ahead of print. ABSTRACT PURPOSE: Bispecific antibodies (BsAbs) directed against B-cell maturation antigen (BCMA; teclistamab) or the orphan G protein-coupled receptor GPRC5D (talquetamab) induce deep and durable responses in...
Targeting myeloma essential genes using NOT Gated CAR T-cells, a computational approach
Leukemia. 2024 Apr 30. doi: 10.1038/s41375-024-02247-1. Online ahead of print. NO ABSTRACT PMID:38688994 | DOI:10.1038/s41375-024-02247-1
Complex Pathological Femoral Fracture in a Multiple Myeloma Patient Undergoing Intertrochanteric Fixation: A Case Report
Cureus. 2024 Apr 14;16(4):e58224. doi: 10.7759/cureus.58224. eCollection 2024 Apr. ABSTRACT Pathological fractures commonly occur in patients with metastatic bone diseases, particularly multiple myeloma. The current optimal management for metastatic pathological lesions affecting the proximal...
A comparative analysis of transcriptomics of newly diagnosed multiple myeloma: exploring drug repurposing
Front Oncol. 2024 Apr 16;14:1390105. doi: 10.3389/fonc.2024.1390105. eCollection 2024. ABSTRACT Multiple myeloma (MM) is an incurable malignant plasma cell disorder characterized by the infiltration of clonal plasma cells in the bone marrow compartment. Gene Expression Profiling (GEP) has emerged...
Expert Recommendations & Patient Perspectives for Treating Relapsed/Refractory Multiple Myeloma
In this webcast, expert faculty discuss best practices in treating patients with relapsed/refractory multiple myeloma and discuss the patient's perspective on treatment options. Learners will also get a look ahead at emerging therapeutic strategies and clinical trials...
Overview of Key Early-phase CAR T-cell Therapy Studies in Relapsed or Refractory Multiple Myeloma
In this article, we look at some of the early-phase clinical trials, where chimeric antigen receptor (CAR) T-cell therapy has been targeted to the B-cell maturation antigen (BCMA) in patients with relapsed or refractory MM (RRMM). The anti-BCMA CAR T-cell...
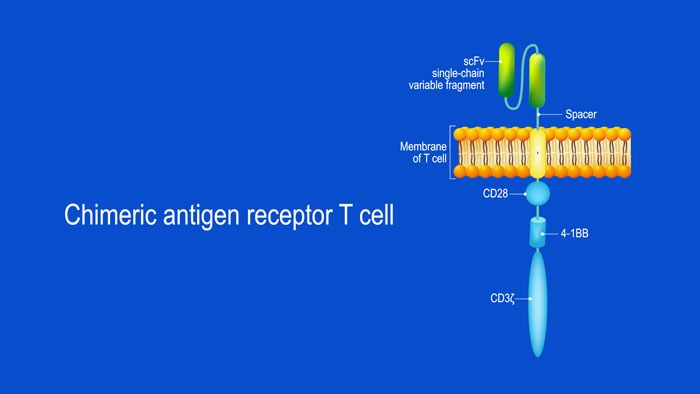
What Are Chimeric Antigen Receptor (CAR) T-cells and What is Their Potential Target Site in Multiple Myeloma?
CAR T-cell structure CARs are typically composed of four regions: (1) an extracellular antigen-binding domain; (2) a hinge or spacer peptide; (3) a transmembrane domain that anchors the CAR to the cell membrane; and (4) one or more intracellular signalling domains...